Probleemstelling:
Cerebral Arteriovenous Malformation (AVM) is an anomaly (abnormality) with a direct connection between arteries and veins (see Fig 1a), which causes the vessels to intertwine (they make a bundle), having a high probability of hemorrhage (they burst and cause stroke- bleeding of brain blood vessels). To prevent bleeding, an embolization (a procedure to insert glue and coils into the AVM using a catheter to prevent it from bursting) is performed. The main problem is that only the highly bundeled part of the AVM, called the nidus, needs to be filled, while the vein (which drains the blood from the AVM) must remain free. In other words, we must separate the AVM into its main components: arteries (feeding the AVM with blood), veins (draining blood from the AVM) and the nidus (to be filled with coils) (see Fig 1b). One way to perform this is to use image segmentation and centerline-extraction algorithms:
http://telin.ugent.be/~dbabin/Skeletonization02.avi
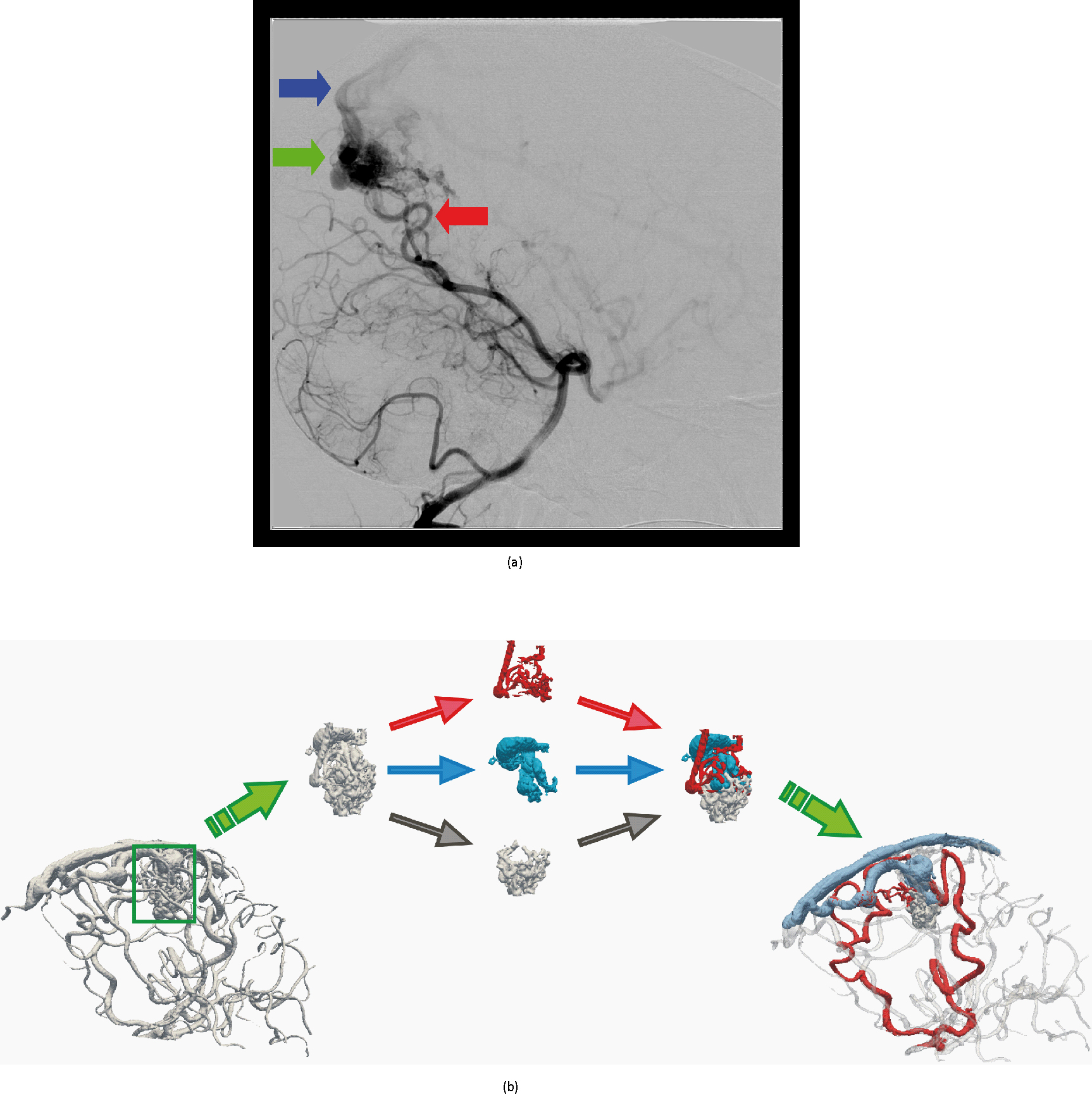
Fig. 1. (a) Digital Subtraction Angiography (DSA) image of cerebral blood vessels, which is used to navigate the catheter through the blood vessels in embolization procedures. Red arrow show the arteries, green the AVM and blue the vein. (b) Illustration of AVM decomposition into arteries (red), veins (blue) and the nidus (gray).
These methods perform AVM decomposition based on vessel properties (radii, vessel positions). However, the main problem is that these methods do not give any information on blood flow directions and intensities inside the AVM, which is of utmost importance in order to successfully navigate the catheter and perform embolization.
Another important cardiovascular health problem is atrial fibrilation (irregular heart function) which causes creation of blood clot (coagulated blood) in the left atrial appendage (LAA, a small "baggy" chamber attached to the left atrium of the heart). In time, the chaotic rhythm may cause blood to pool in the LAA and form clots. If a blood clot forms, it could dislodge from the LAA and travel to the brain. There it might block blood flow, causing a stroke. For this reason, the LAA is often blocked using an occluder to prevent the movement of the clotted blod. Left atrial appendage (LAA) can have various shapes (see Fig. 2), which yield different clotting scenarios. In other words, depending on the size and shape the LAA may produce more or less blood clots. Analysis of the blood flow in the LAA will give vital information on the risk of the blood clot forming in the specific LAA shape.

Fir. 2. Various shapes of the left atrial appendage (LAA) marked with long arrows.