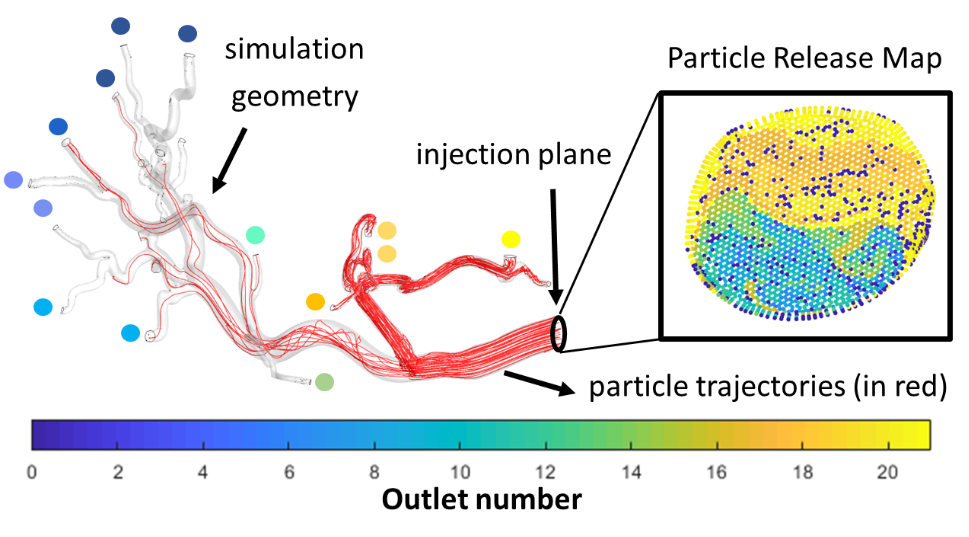
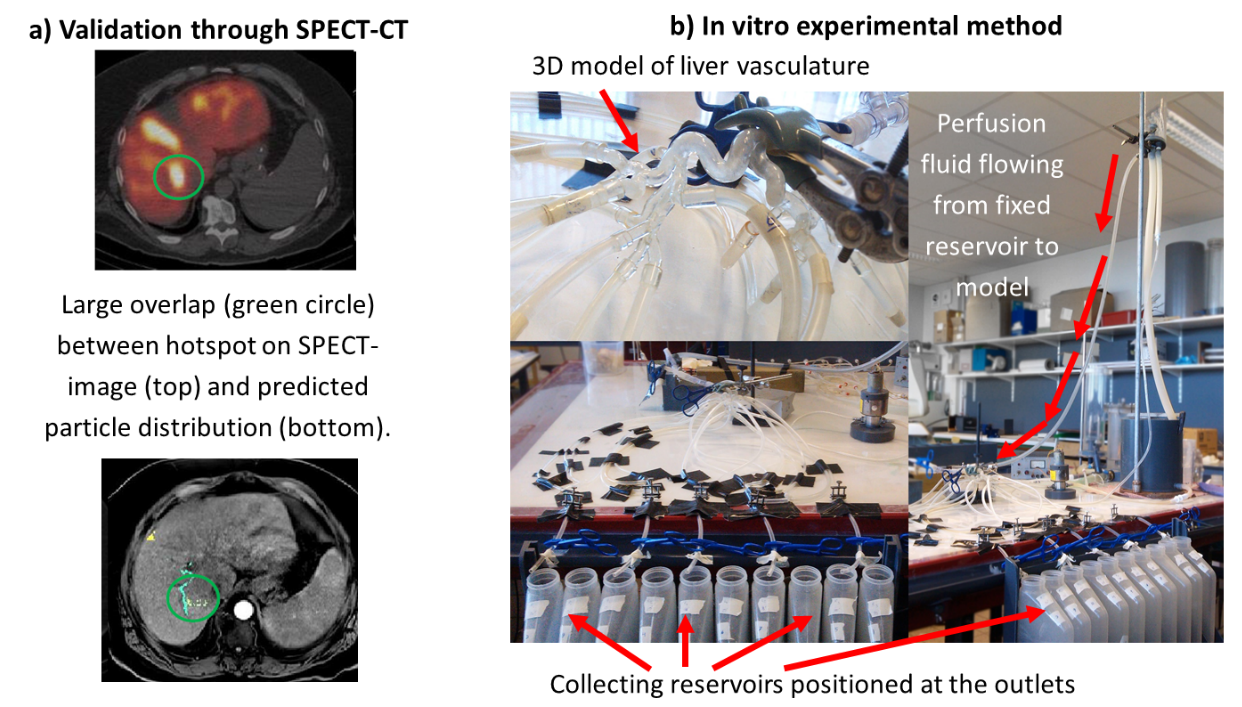
Probleemstelling:
THE DISEASE: LIVER CANCER
Hepatocellular carcinoma (HCC) is the most common form of primary liver cancer and the second-highest leading cause of cancer-related deaths. Effective and site-specific strategies for the administration of therapeutic drugs are urgently needed, especially for patients with unresectable tumors.
THE TREATMENT: TRANSARTERIAL THERAPIES
Unresectable HCC can be treated by transarterial chemoembolization (TACE) or radioembolization (TARE). During these therapies, the patient is catheterized through the femoral artery and the catheter is advanced via the aorta towards the liver (Figure 1, panels a-b). Microspheres are injected in the proper hepatic artery or, ideally, closer to the tumor (Figure 1, panel c). In TACE, these microspheres carry drug particles and are used to permanently damage the tumor tissue by a combined embolic (occlusive) and chemotherapeutic effect. In TARE, the embolic effect is only of secondary importance- the main pathway by which tumor tissue is damaged, is through local emission of high-intensity radiation.
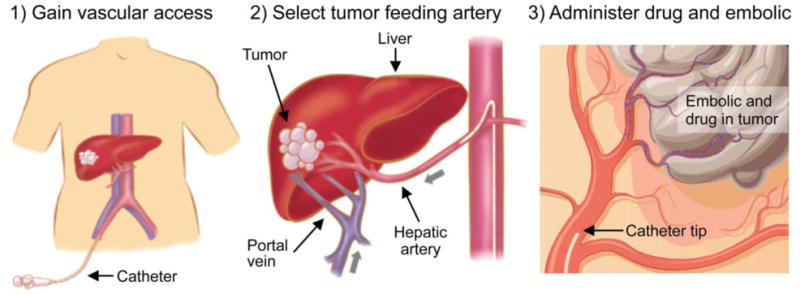
Figure 1: The workflow for transarterial therapies (TACE, TARE). A catheter is inserted in the femoral artery and navigated towards the liver. Damaging microspheres are injected as close as possible to the tumor to maximize the target-specificity of the treatment.
THE GOAL: EFFICIENT & SPECIFIC DRUG DELIVERY
The overall goal is to steer the damaging microspheres towards the tumor tissue to (i) maximize drug delivery to the tumor, and (ii) limit the amount of toxicity delivered to healthy tissue. This method of delivery for these transarterial therapies stand out against unspecific drug delivery, such as conventional intravenous chemotherapy, where drugs pass through the systemic circulation and severe side-effects (e.g. nausea, hair loss) may occur as a result of unwanted toxicity at the level of the healthy tissue.
THE QUESTION: CAN WE STEER PARTICLES TOWARDS THE TUMOR?
Because it is often challenging to navigate catheters inside the smaller arteries and release particles close to the tumor, easily accessible, upstream locations are instead used to release particles. There, the problem arises how microspheres can be steered through the bloodstream towards the tumor. A range of parameters that are variable in clinical practice (e.g. particle size, particle density, injection velocity, injection location, catheter type, catheter tip orientation, etc.) may have a large impact on where particles eventually end up. However, as of now, this impact is unclear. A limited range of studies in simplified geometries have been performed, but the impact is likely to depend highly on the patient-specific geometry used.
If certain parameters prove to be highly impactful on the downstream particle distribution, they can be used in a patient-specific optimization workflow to increase the target-specificity of the eventual treatment (e.g. selecting the most optimal injection location and catheter type for each patient independently.)