Probleemstelling:
THE PROMISE OF WHOLE ORGAN BIOENGINEERING
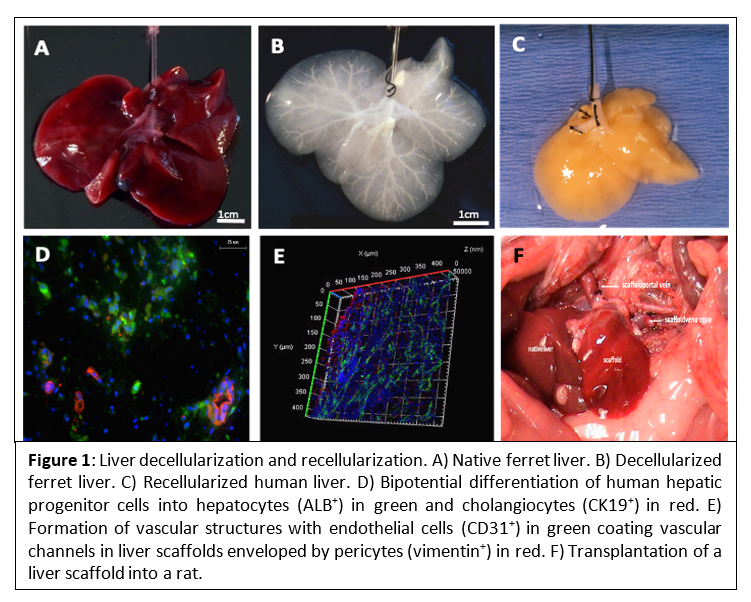
Organ decellularization, followed by recellularization, has established itself in the last decade as a powerful and promising technology to generate solid organs for transplantation, hopefully alleviating the dire shortage of organ donors in the coming future (Figure 1)(1–3). However, major challenges remain unresolved. Namely, the recellularization of liver decellularized scaffolds is still quite varied, which makes their full revascularization difficult and heterogeneous(4).
NEED FOR OPTIMIZATION OF RECELLULARIZATION PROTOCOLS
Hence, optimization of recellularization/revascularization conditions has been targeted by several research groups, including Baptista´s Laboratory (University of Zaragoza, Spain)(5). By using differential pressures of cell perfusion, Baptista’s Lab is now able to target vascular structures with different diameters, enabling the recellularization of the whole vascular tree (in preparation). However, this process needs further optimization to ensure full coverage of the whole vascular network.
NUMERICAL FLUID SIMULATIONS AS AN OPTIMIZATION TOOL?
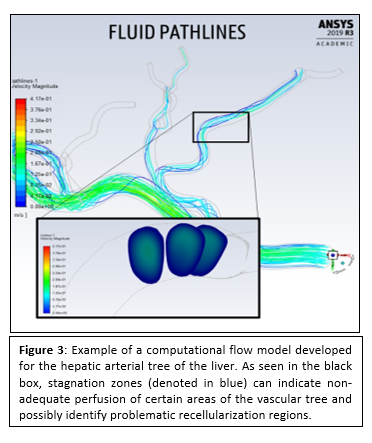
The question remains whether we can navigate the perfused cells to their intended locations and coax them to form mature vascular structures. Therefore, in silico (i.e. computer) simulations of cell penetration, distribution and organization might help determining the right parameters to answer this question and homogeneously recellularize these scaffolds. Hence, we propose to use Computational Fluid Dynamics (CFD) to determine initial cell distribution and penetration according with the perfusion pressures used.
VALIDATING NUMERICAL SIMULATIONS WITH EXPERIMENTAL DATA
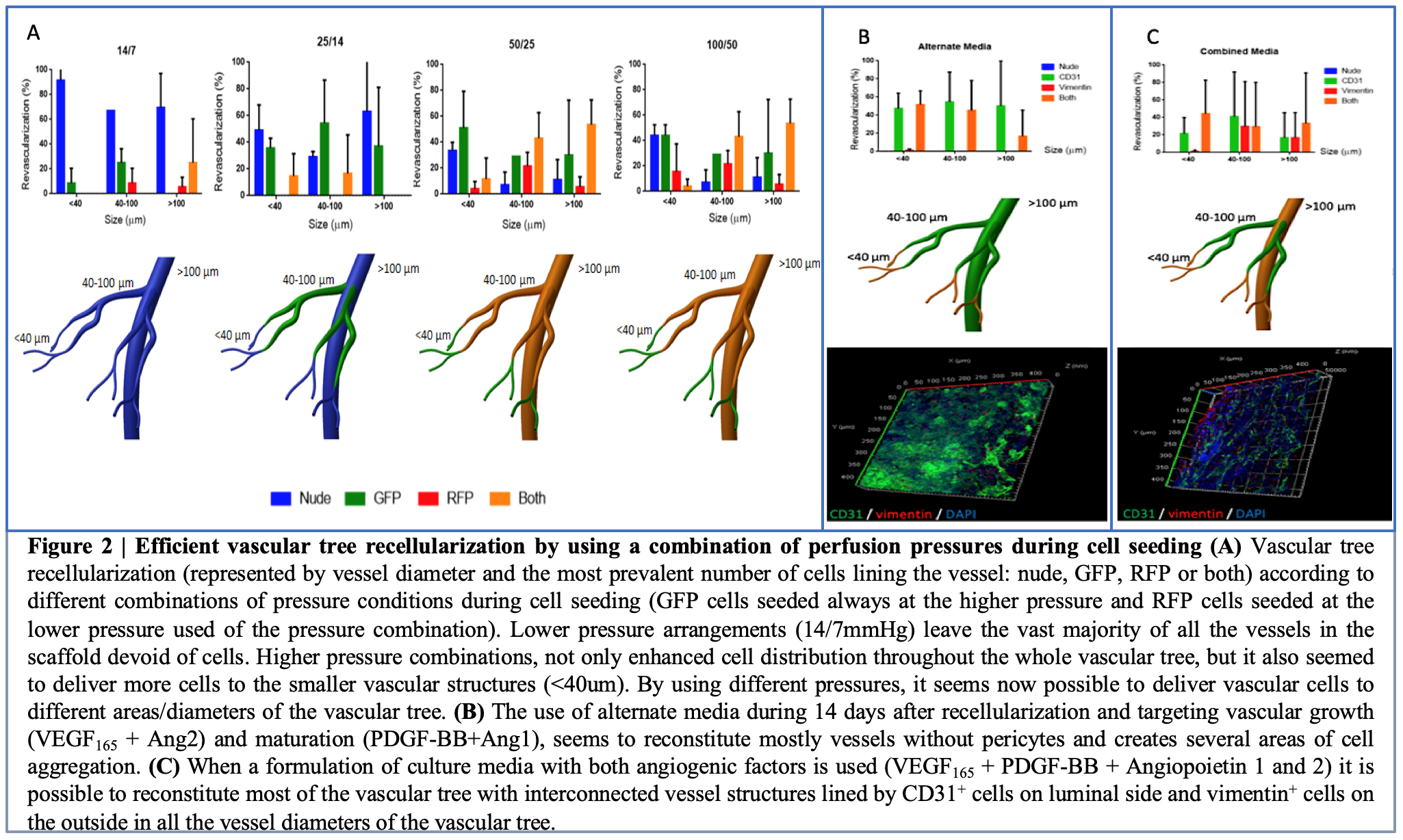
Preliminary work performed by Baptista’s Lab has showed that by using higher pressure combinations to recellularize these scaffolds it is possible to almost completely coat large to small diameter vascular structures with the necessary cells (Figure 2). These experimental data on recellularization pressures and cell localization will be used to developed the in-silico model for cell penetration, distribution and organization.
References
1. Baptista PM, Siddiqui MM, Lozier G, Rodriguez SR, Atala A, Soker S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53(2).
2. Moran EC, Dhal A, Vyas D, Lanas A, Soker S, Baptista PM. Whole-organ bioengineering: Current tales of modern alchemy. Transl Res. 2014;163(4).
3. Takeishi K, Collin de l’Hortet A, Wang Y, Handa K, Guzman-Lepe J, Matsubara K, et al. Assembly and Function of a Bioengineered Human Liver for Transplantation Generated Solely from Induced Pluripotent Stem Cells. Cell Rep. 2020;31(9).
4. Baptista PM, Atala A. Regenerative Medicine: The Hurdles and Hopes. Translating Regenerative Medicine to the Clinic. 2015.
5. Baptista PM, Moran EC, Vyas D, Ribeiro MH, Atala A, Sparks JL, et al. Fluid flow regulation of revascularization and cellular organization in a bioengineered liver platform. Tissue Eng - Part C Methods. 2016;22(3).