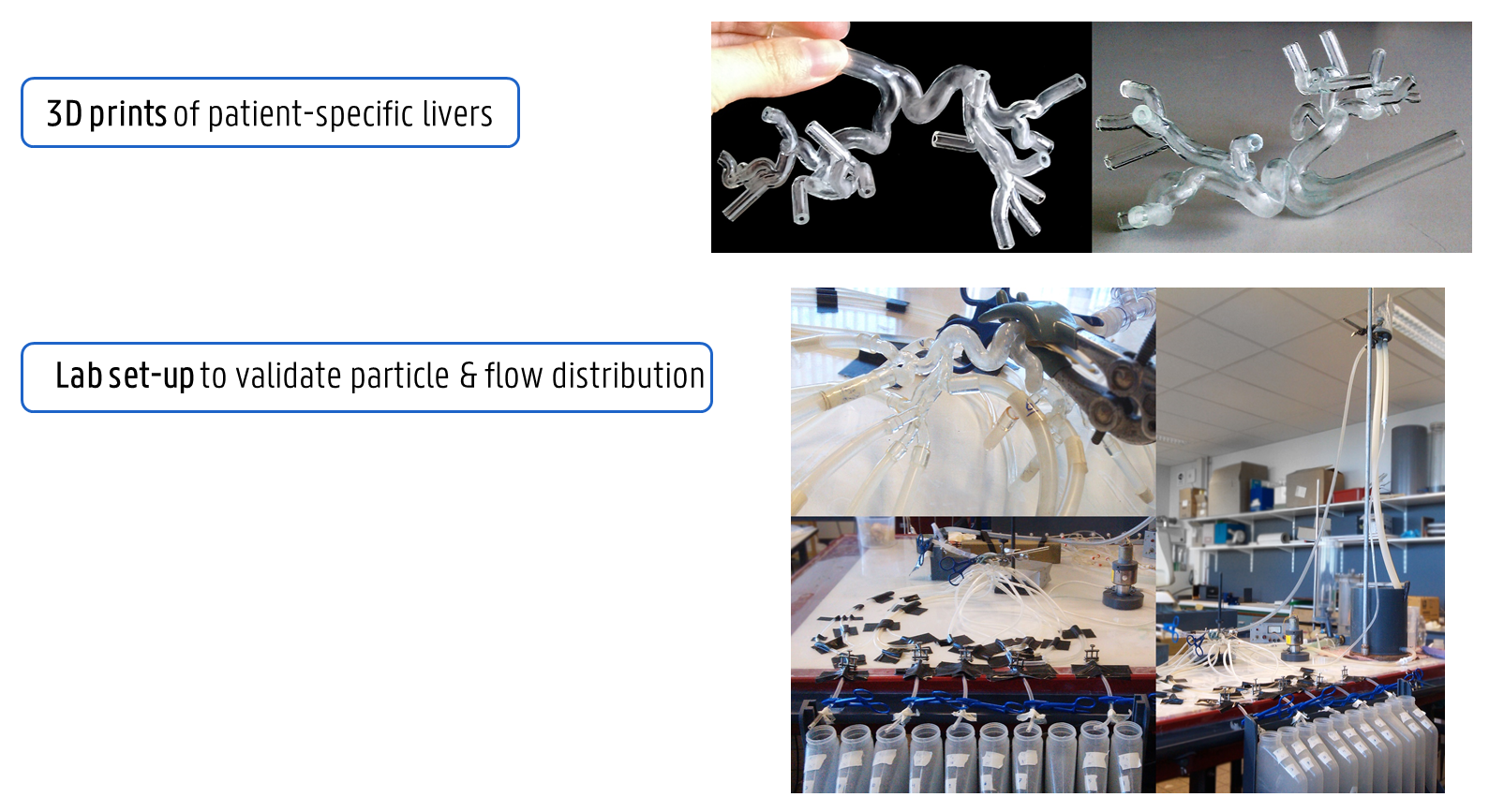
Probleemstelling:
CURRENT SHORTCOMINGS: SUBOPTIMAL TREATMENTS FOR LIVER CANCER
Hepatocellular carcinoma (HCC) is the most common form of primary liver cancer, ranking fourth in mortality worldwide. At its intermediate, unresectable stage, HCC can be treated by transarterial chemoembolization (TACE) or radioembolization (TARE). During these therapies, catheters are retrogradely advanced through the femoral artery to the hepatic arteries (Fig. 1, panels 1&2), where embolizing microspheres are locally injected to selectively damage tumor tissue (Fig. 1, panel 3). The overall goal is to steer the damaging microspheres towards the tumor tissue to (i) maximize drug delivery to the tumor, and (ii) limit the amount of toxicity delivered to healthy tissue. However, the execution of these procedures depends on the implementation of several clinically variable parameters: the axial injection location (e.g. close to the tumor or more upstream), the catheter type (e.g. fixed vs variable tip orientation), the microsphere type (different sizes and densities), etc. Since some patients respond well to the treatment and others do not, it is currently unclear to which extent these treatment heterogeneities contribute to suboptimal outcomes in some patients.
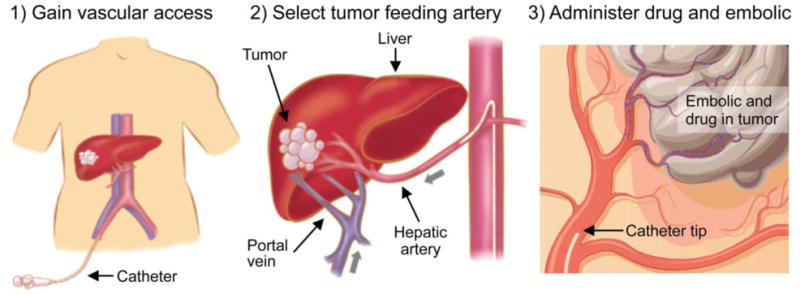
Figure 1. The workflow for transarterial therapies (TACE, TARE). A catheter is inserted in the femoral artery and navigated towards the liver. Damaging microspheres are injected as close as possible to the tumor to maximize the target-specificity of the treatment.
RESEARCH GOAL: USING COMPUTER MODELS TO EVALUATE MEDICAL DEVICES
Therefore, computational fluid dynamics (CFD) modelling has been applied to elucidate the role of these clinical parameters. For example, CFD simulations of blood and microparticle flow in the hepatic arteries have already shown that the injection location and timing can have a large impact on the downstream particle distribution. Additionally, CFD simulations can be used to evaluate the impact of using different catheter types (see Fig. 2 for an example). As of now, multiple catheter types are commercially available, but the direct impact of these different designs is currently unclear. Relevantly, using computer models to compare different medical devices is already very common in cardiology, but not yet in the liver. Therefore, this thesis will focus on developing computer models of different catheter types in the hepatic arteries, and evaluating their impact on the blood flow and microparticle distribution.
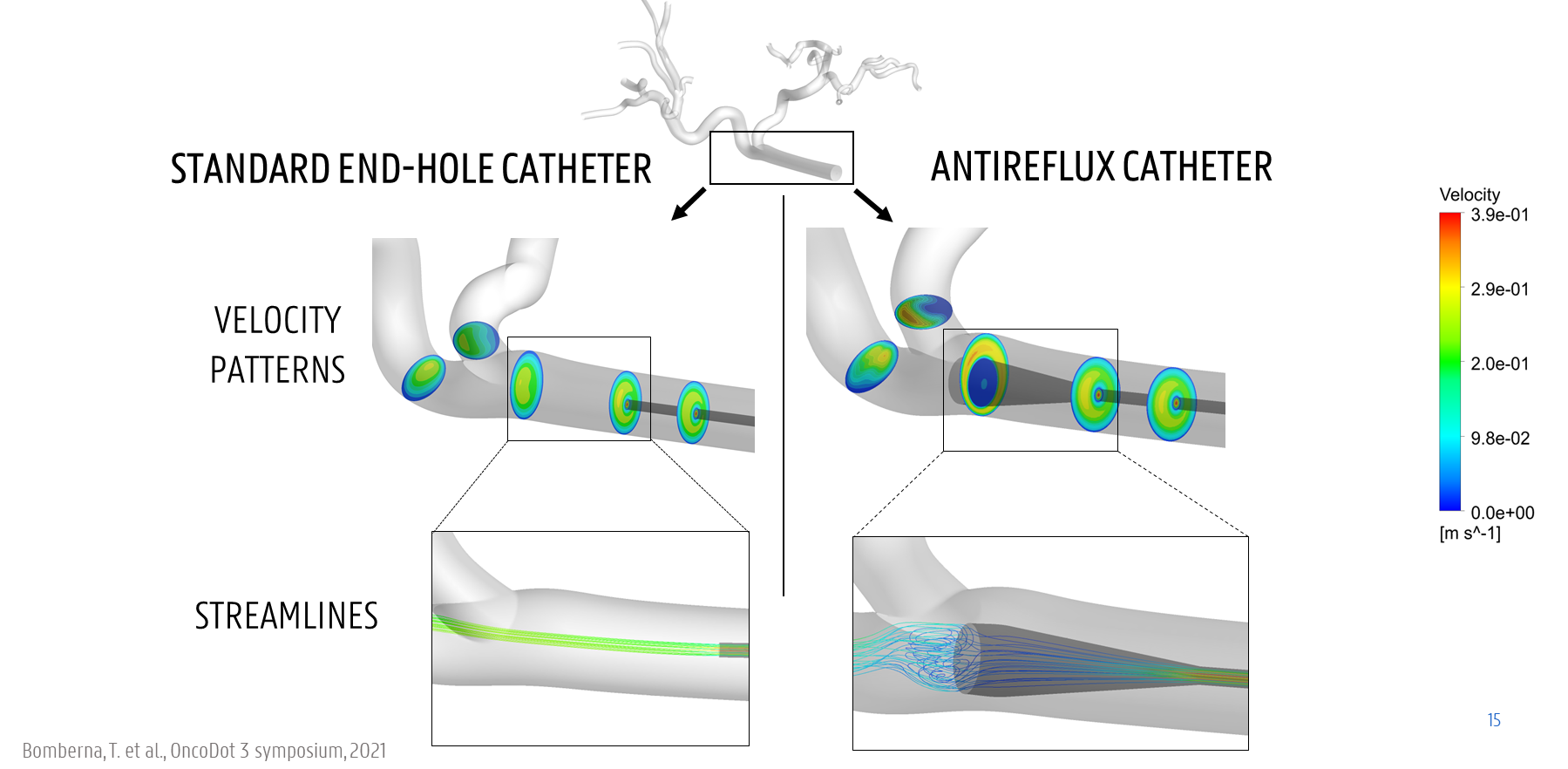
Figure 2. Example of comparing a straight standard microcatheter and a microcatheter with expanded tip in a virtual model of the blood flow.