Doelstelling:
A single-filament (lumen) catheter (Tenckhoff) is currently applied for the intraperitoneal drug delivery by putting it in place operatively deep in the pelvis. Disadvantages of the current catheter design include the inability to reach all affected peritoneal surface areas due to the presence of adhesions, and the short treatment duration. The idea is to find a replacement for the commonly used catheter. Advantages include better exposure of the entire peritoneal surface to the chemotherapeutic drug, the possibility to apply metronomic therapy, and the avoidance of side effects. To reach these goals, we designed a novel branched catheter. In this context, computational simulations and in-vitro experiments are the next steps to be taken to strengthen this project and further optimize the catheter design. More specifically, sensitivity studies are needed to optimize the setup and significant parameters such as the diameters and positions of perforations. Based on initial computational results, a prototype was already assembled and is currently undergoing further testing. The main aim of this project is to finalize the prototype development by performing in-vitro experiments to test/validate the novel branched catheter (see Figure 1).
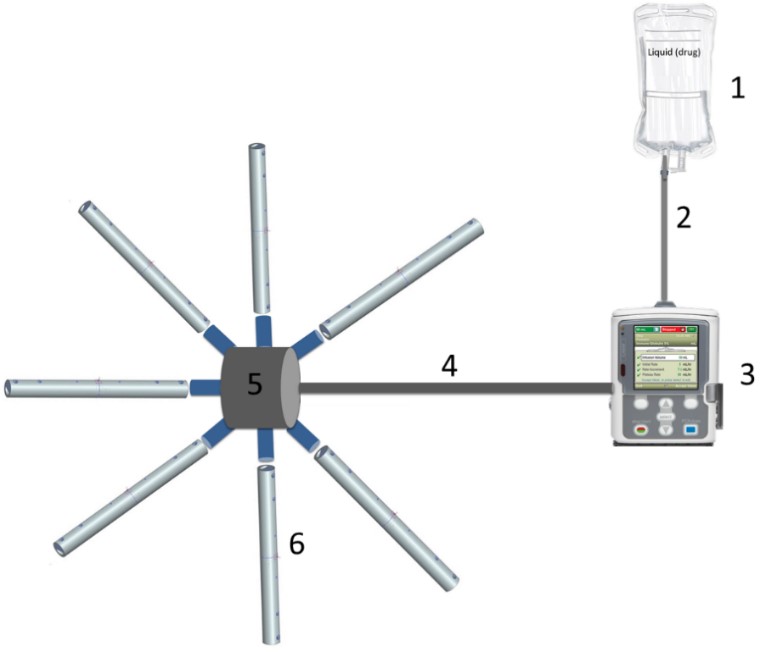
Figure 1. Experimental setup: (1) liquid (drug), (2) connecting tube; (3) infusion pump, (4) transport catheter, (5) distributing connector, (6) perforated catheters.
At the end of this project, the following questions will be answered:
- Are the results of CFD simulations comparable with experimental results?
- How do different parameters of the branched catheter affect the outflow distributions?
- What are the optimized parameters for the catheter?