Probleemstelling:
CLINICAL BACKGROUND: A PROMISING NOVEL TREATMENT FOR LIVER CANCER
Liver cancer, known as hepatocellular carcinoma (HCC), is the third leading cause of cancer-related death worldwide. It is commonly detected at an advanced incurable stage with poor survival outcomes and limited treatment options.
Interestingly, gamma delta T-cells, which are a subpopulation of immune cells found in human blood and tissues, possess strong cancer-killing properties and exciting potential to create a more universally effective cancer immunotherapy. In previous work, we demonstrated at the University of Birmingham that combining in vitro expanded gamma delta T-cells with direct intra-tumoural delivery of the aminobisphosphonate Zoledronate in HCC, led to enhanced local activation of gamma delta T-cells, with a marked increase in tumour cell lysis. We aim to take forward this novel drug delivery and immunotherapy combination strategy to an early-phase clinical trial for patients with liver cancer.
However, intra-tumoural delivery of Zoledronate can only be attained in clinical practice via intra-arterial injection in the hepatic arteries. Several key questions remain regarding this delivery method. These include: i) what proportion of Zoledronate uptake would occur by the HCC tumour compared to neighbouring liver tissue, ii) how well can transarterial delivery of Zoledronate penetrate HCC tumours, and iii) how would patient-specific factors including tumour size and site influence the concentration of Zoledronate in the tumour?
RESEARCH GOAL: PREDICTING TREATMENT EFFICACY
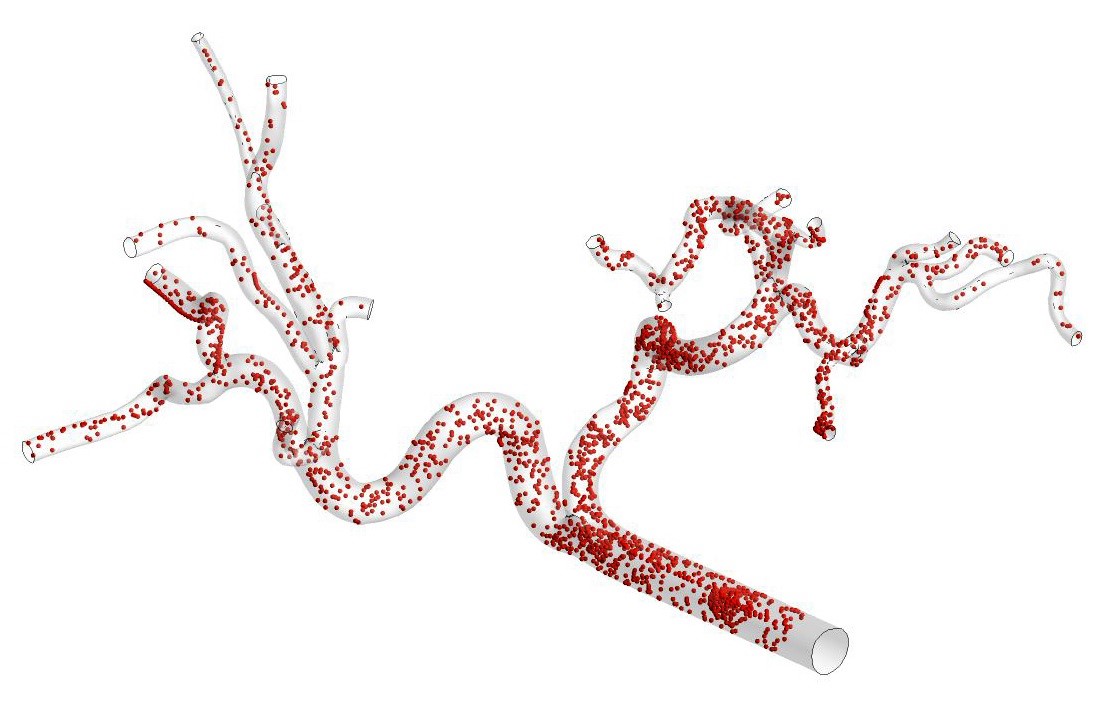
The goal of this project is to use computational fluid dynamics (CFD) simulations to model Zoledronate mass transport through the 3D-reconstructed arterial vasculature of patient-specific livers with HCC tumours, examining the influence of blood flow on drug delivery and delivery to the tumour. This work will extend our ongoing work at UGent focused on CFD modeling of microparticle transport for transarterial radio-embolisation procedures for HCC (see example of a particle distribution in the Figure below). However, for this thesis project, the modeling approach will have to be adapted to model mass transport of Zoledronate instead of microparticles. CFD modelling techniques can also be used to assess the impact of tumour-specific factors including cancer burden and tumour geometry on Zoledronate distribution in the hepatic arteries.
In short, this Master thesis will focus on developing CFD models for optimising delivery strategies (i.e. by adapting injection angle, location) to maximize target site specificity and limit Zoledronate particle spread to surrounding healthy liver tissue. The work from this project has exciting potential to inform the design of an early-phase clinical trial encompassing transarterial Zoledronate delivery for patients with HCC, and could be used to predict treatment efficacy.